Interstitial Fluid Pressure
Model Summary Jain et al. Cancer Research 2007; 67:2729-2735
Preclinical and clinical evidence shows that anti-angiogenic agents can decrease tumor vessel permeability and interstitial fluid pressure (IFP) in a process of vessel “normalization.” The resulting normalized vasculature has more efficient perfusion, but little is known about how tumor IFP and IFV (interstitial fluid velocity) are affected by changes in transport properties of the vessels and interstitium that are associated with anti-angiogenic therapy.
By utilizing a mathematical model to simulate IFP and IFV profiles in tumors (Jain and Baxter, Cancer Research 1988), we have shown that anti-angiogenic therapy can decrease IFP by decreasing the tumor size, vascular hydraulic permeability and/or the surface area per unit tissue volume of tumor vessels (Figures 3, 4). Within a certain window of anti-angiogenic effects, interstitial convection within the tumor can increase dramatically, while fluid convection out of the tumor margin decreases (Figure 4). This would result in increased drug convection within the tumor and decreased convection of drugs, growth factors, or metastatic cancer cells from the tumor margin into the peri-tumor fluid or tissue. Decreased convection of growth factors, such as VEGF-C, would limit peri-tumor hyperplasia and decreased VEGF-A would limit angiogenesis in sentinel lymph nodes. Both of these effects would reduce the probability of lymphatic metastasis. Finally, decreased fluid convection into the peri-tumor tissue would decrease peri-tumor edema associated with brain tumors and ascites accumulation in the peritoneal or pleural cavity– a major complication with a number of malignancies (Jain et al., Cancer Research, 2007).
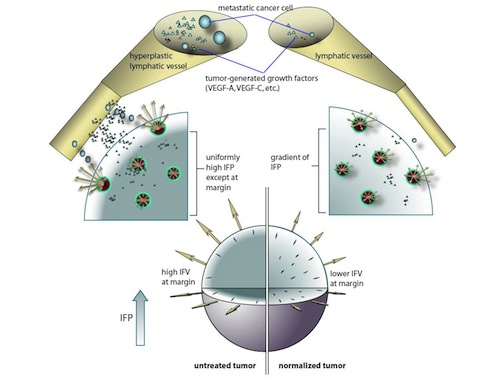
Jain et al. Cancer Research 2007; 67:2729-2735
The tumor is modeled as a sphere of radius R, embedded in a body fluid (e.g, peritoneal cavity, pleural cavity) or host tissue. The interstitial fluid oozing from the tumor periphery carries therapeutics (e.g, monoclonal antibodies), growth factors (e.g, VEGF-A, -B, -C and -D) and cells (e.g., metastatic cells) into the surrounding fluid/tissue. The result may be higher fluxes of cancer cells and growth factors from the tumor into the surrounding tissue or body fluid. The growth factors (including VEGF-A, B, C and D) can induce angiogenesis and lymphangiogenesis and the cancer cells can contribute to metastatic dissemination via the lymphatic or blood vessels. Fluid seeping from the tumor surface can also cause edema (e.g. around brain tumors) and ascites formation (e.g. ovarian cancer). Anti-angiogenic therapy can “normalize” the tumor vasculature, leading to lower IFP at the center, less steep IFP gradients and lower fluid flow rates at the tumor margin, thus potentially reducing peri-tumor edema, ascites formation and lymphatic metastasis. Furthermore, the normalized vessels may be more resistant to cancer cell intravasation, a prerequisite for hematogenous metastasis (Jain et al., Cancer Research, 2007).
