Lance Munn
Munn Lab Research
Tumor mechanobiology and stress responses
Tumor growth alters the chemical and mechanical environment, exposing cancer cells to many types of stress. We are investigating how mechanical forces and chemical stresses affect tumor mechanobiology and stress granule biology.
Anti-cancer immunity
Initiation of an immune response requires activation of one or more naïve T cells, which move between lymph nodes searching for matching antigen. If a match is made, the T cell activates, proliferates and enters the circulation in search of the source of antigen. Little is known about this process, especially in the context of anti-cancer immunity and immune checkpoint programs that quench the immune response. We are developing computational models in conjunction with experimental models of immune cell trafficking, lymphatic function and tumor growth to study this problem.
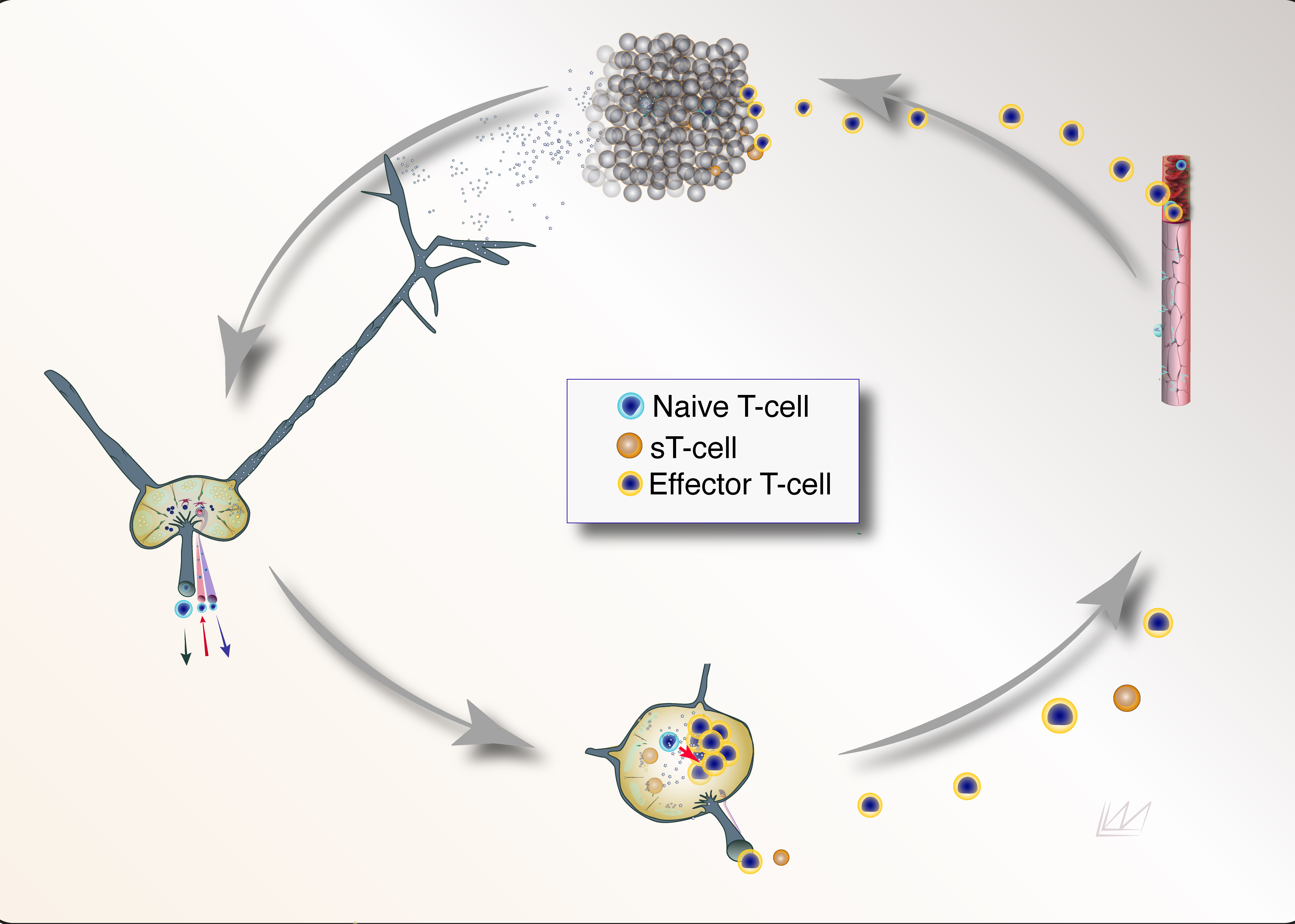
Tumor Tissue Engineering
Our understanding of cancer progression or response to therapies would benefit from easily accessible, tissue-level assays that recapitulate the biology and anatomy of human tumors. To this end, we have developed methodology for integrating tumor explants with extended stroma and vasculature in vitro. With appropriate culture conditions, a self-assembled vascular network develops and then incorporates into co-cultured tumors excised from mice or patients. Using this approach, we are creating a platform for optimally maintaining samples from patients for the purpose of biological analysis and drug testing.
Lymphatic pumping
Flow of fluid within the lymphatic system is central to many aspects of physiology, including fluid homeostasis and immune function, and poor lymphatic drainage results in significant morbidity in millions of patients each year. We are investigating the mechanisms of lymphatic pumping, considering the nitric oxide and calcium dynamics driven by mechanobiological mechanisms.

Angiogenic sprouting
During angiogenesis, endothelial cells abandon their normal arrangement in the vessel wall to migrate into the extravascular matrix. This process is controlled by multiple signals and is necessary for tissue regeneration and tumor growth. Using in vitro models and microfluidic devices, we are investigating the biochemical and mechanical determinants of this morphogenic transformation.
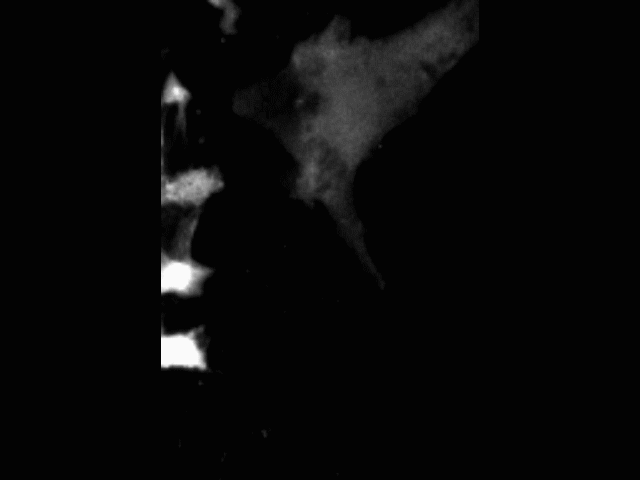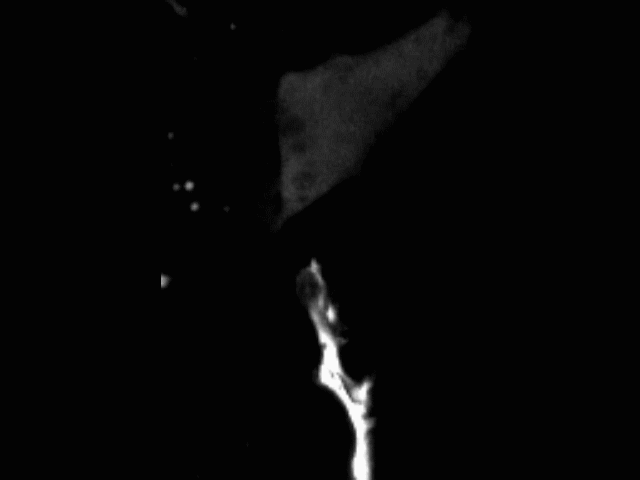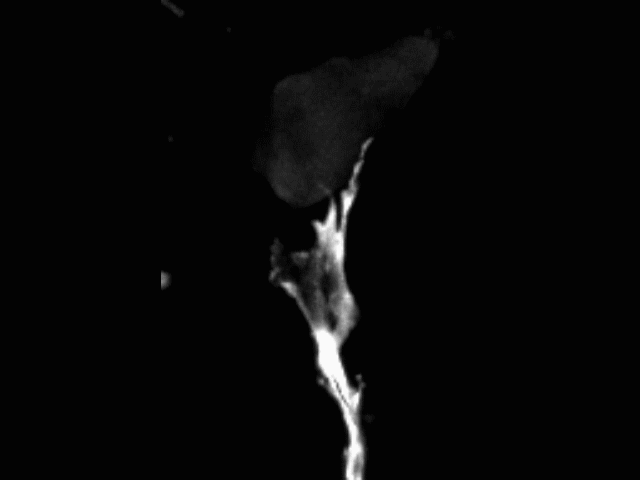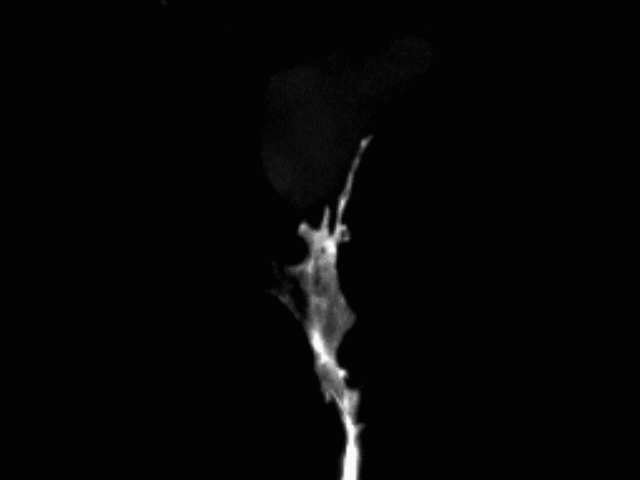
Vascular anastomosis
To form new, patent blood vessels, angiogenic sprouts must connect. The process by which this happens — anastomosis — is poorly understood, but represents new targets for vascular therapy. Using intravital microscopy and engineered vascular devices, we are following the steps of anastomosis to identify cellular and molecular mechanisms that may eventually be targeted for enhancing wound healing or inhibiting pathological angiogenesis.
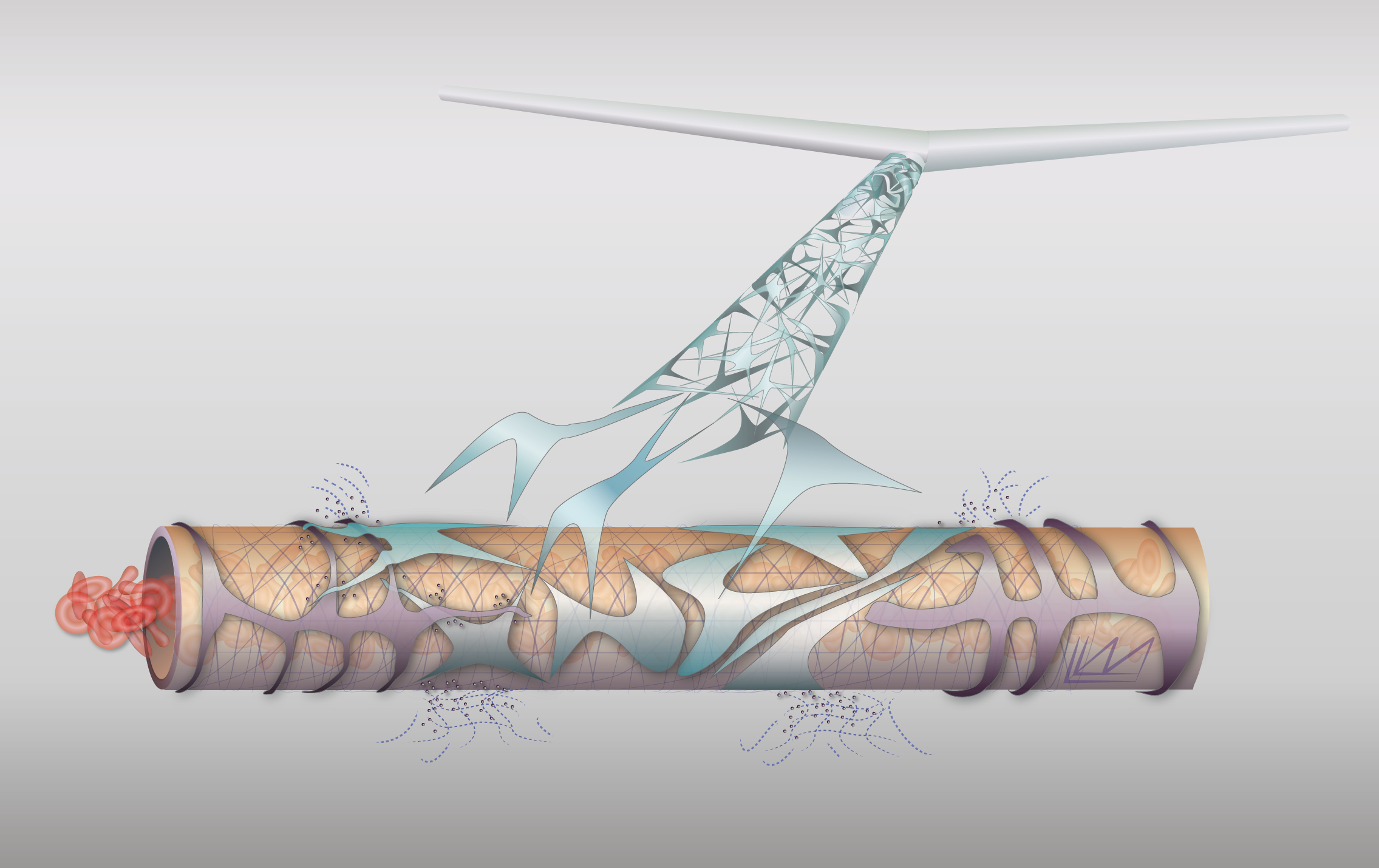
Blood vessel remodeling
In many normal physiological responses, endothelial cells and the blood vessel networks they form undergo dramatic changes in morphology and function. Examples include angiogenesis in wound healing, vessel dilation/hyperpermeability in inflammation, and endometrial angiogenesis in the female reproductive cycle. Endothelial cells, in cooperation with other stromal cells, have to accomplish these diverse changes by responding to a limited number of growth factors including VEGF, PlGF and bFGF. We are using a systems biology approach to understand how the various growth factors and cells cooperate to produce these seemingly diverse functions. Because tumor angiogenesis relies on many of these same growth factors and cellular mechanisms (but in an abnormal, poorly controlled way), these studies will allow a better understanding of tumor angiogenesis and anti-angiogenic therapy.
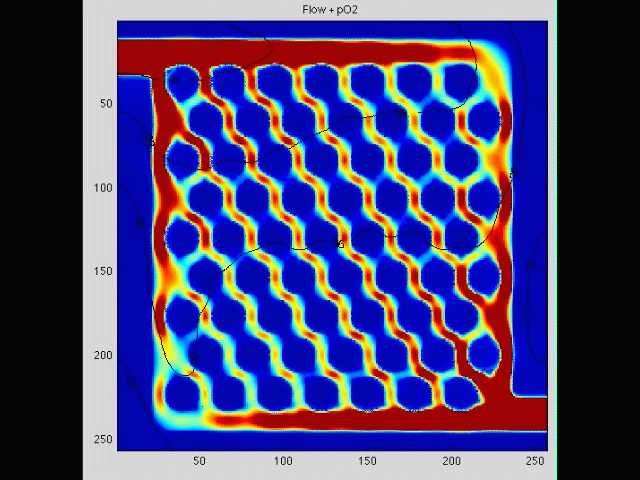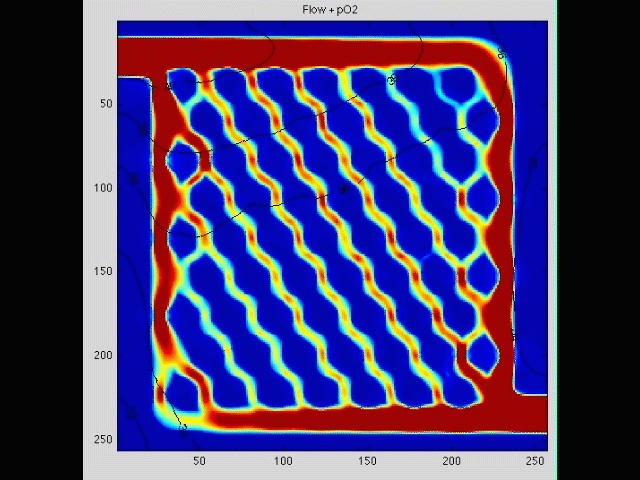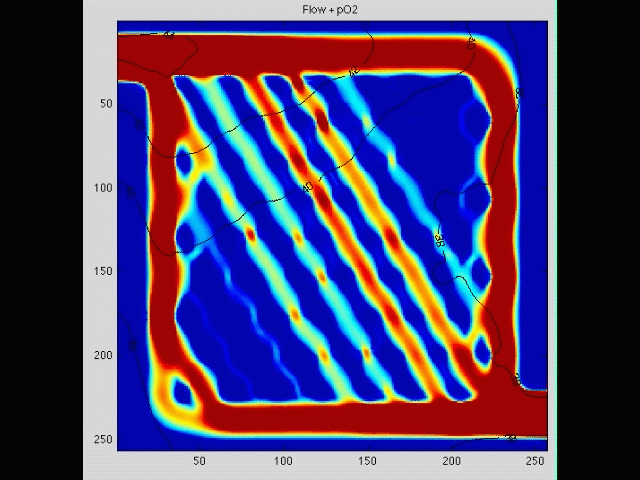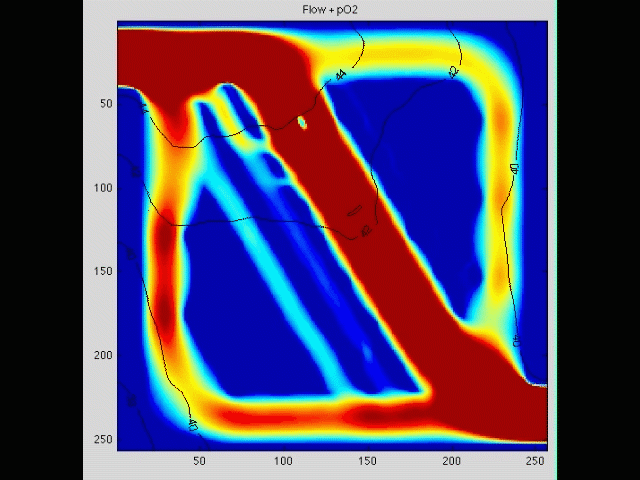
Cancer cell invasion
During the initial stage of metastasis, cancer cells must breach the vessel wall and enter the circulation. Despite intense research in this area, the cellular mechanisms by which this occurs are poorly understood. Some tumors seem to metastasize as single rogue cells, while others travel in groups or clusters; some seem to actively migrate into the vessel, while others may be passively pushed. Using gene array analysis and carefully designed coculture systems, we are assessing the mechanical and cellular determinants of the initiation of metastasis.
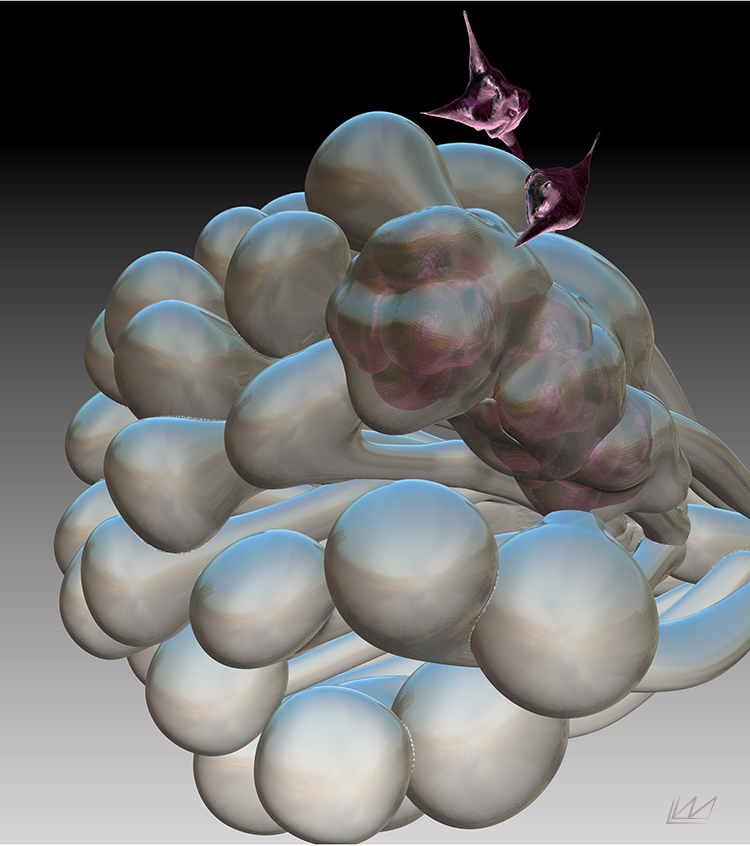
Mathematical modeling
With sufficient understanding of the underlying mechanisms, mathematical models can be assembled to validate existing hypotheses and generate new ones.

Lab News
"CAR-T cells are T cells that have been extracted from a patient, modified in a laboratory to recognize and target cancer cells then infused back into the patient. They have revolutionized the treatment of many blood cancers, but have not yet benefited patients with solid tumors, such as lung, breast, colorectal or brain tumors. By developing a sophisticated mathematical model, we demonstrated that normalizing tumor blood vessels — which means transforming the abnormal, disorganized vasculature of a tumor into a more normal, structured and functional state — can improve the efficacy of CAR-T therapy and reduce the required therapeutic dose by about fivefold."
Brain tumor patients with glioblastoma (GBM) face extremely limited treatment options and show poor responses to current immunotherapies. New treatment strategies are desperately needed.
Wnt signaling is a crucial cell communication process that regulates various aspects of stem cell behavior. It is also pivotal in the generation of GBM and contributes to treatment resistance.
In this new study, we identify Wnt7b, which is highly expressed in GBM patients, as a previously unknown determinant of resistance to immune checkpoint blockers.
The physical microenvironment plays a crucial role in tumor development, progression, metastasis and treatment. Recently, we proposed four physical hallmarks of cancer, with distinct origins and consequences, to characterize abnormalities in the physical tumor microenvironment: (1) elevated compressive–tensile solid stresses, (2) elevated interstitial fluid pressure and the resulting interstitial fluid flow, (3) altered material properties (for example, increased tissue stiffness) and (4) altered physical micro-architecture. As this emerging field of physical oncology is being advanced by tumor biologists, cell and developmental biologists, engineers, physicists and oncologists, there is a critical need for model systems and measurement tools to mechanistically probe these physical hallmarks. Here, after briefly defining these physical hallmarks, we discuss the tools and model systems available for probing each hallmark in vitro, ex vivo, in vivo and in clinical settings. We finally review the unmet needs for mechanistic probing of the physical hallmarks of tumors and discuss the challenges and unanswered questions associated with each hallmark.
Current SARS-CoV-2 vaccines are effective at preventing COVID-19 or limiting disease severity in healthy individuals, but effectiveness is lower among patients with cancer or immunosuppression. A team led by Drs. Jain, Stylianopoulos and Munn predict vaccine effectiveness over time by building a mathematical framework to account for vaccination-induced immunity. A booster dose of both mRNA vaccines can induce a robust enhancement of both antibody levels and numbers of pertinent types of adaptive immune cells, which is predicted to provide sufficient protection for more than 1 y in healthy patients. However, the model suggests that for immunosuppressed people or patients with cancer receiving an immunosuppressive treatment, the booster effect may wane and should be given boosters on a more frequent basis.
- A mathematical model revealed that the optimal time to initiate immune-modulating therapy in COVID-19 differed according to patients’ medical history and risk factors. Different patients also required different types of immunomodulation for optimal therapy.
- Certain biological markers that differed based on patient characteristics determined optimal treatment initiation time, and these markers pointed to particular biologic programs or mechanisms that affected a patient’s outcome.
- Use of the model may help physicians tailor treatments to different patients and also indicate which patients are most likely to respond to certain drugs tested in clinical trials.
- Press release
