Rakesh Jain
Jain Lab Research
For four decades, my research has focused on one challenge: improving the delivery and efficacy of anti-cancer therapeutics by normalizing the tumor microenvironment. Working on the hypothesis that the abnormal tumor microenvironment fuels tumor progression and treatment resistance, we developed an array of sophisticated imaging technologies and animal models as well as mathematical models to unravel the complex biology of tumors. Using these tools, we demonstrated that the blood and lymphatic vasculature, fibroblasts, immune cells and the extracellular matrix associated with tumors are abnormal, and these collaborate together to create a hostile tumor microenvironment characterized by hypoxia, low pH and high interstitial fluid pressure and solid stress. We next hypothesized that agents that induce “normalization” of the microenvironment can improve the treatment outcome. Indeed, we demonstrated that judicious use of antiangiogenic agents—originally designed to starve tumors—could transiently “normalize” tumor vasculature, alleviate hypoxia, increase delivery of drugs and anti-tumor immune cells, and improve the outcome of various therapies, including immunotherapy (Science 2005, 2019, 2020). In parallel, we provided compelling evidence for vascular normalization in cancer patients treated with antiangiogenic agents. In fact, vascular normalization and the resultant improvement in tumor perfusion and oxygenation associated with longer survival in patients (J Clinical Oncology 2013; Cancer Cell 2014; PNAS 2015). Our preclinical finding that vascular normalization can improve immunotherapy (PNAS 2012) was confirmed by others in randomized phase III trials on combining antiangiogenic therapy with immune-checkpoint inhibitors for lung, kidney, liver and endometrial cancers (New England J Medicine 2018, 2019, 2020), and led to the FDA approvals of six such combinations of antiangiogenic therapy and immune-checkpoint inhibitors for these cancers (Science 2019).
The normalization hypothesis also opened doors to treating various non-malignant diseases characterized by abnormal vasculature that afflict >500 million people worldwide, such as, tuberculosis (PNAS 2015) and neurofibromatosis-2 (NF2) (New England J. Medicine 2009). Based on our findings, bevacizumab was approved for NF2-schwannoma patients in UK in 2014. This hypothesis has also been validated by a number of laboratories worldwide and has changed the thinking about how antiangiogenic agents work alone and in combination with conventional and emerging therapeutics (Science 2005; New England J. Medicine 2009; Nature Rev Drug Discovery 2011; Physiological Rev 2011; Cancer Cell 2014; Nature Reviews Clinical Oncology 2018; Science 2019).
Finally, we discovered that anti-hypertensive drugs capable of “normalizing” the tumor matrix and stromal cells can reprogram the tumor microenvironment to an immunostimulatory milieu and improve the delivery and efficacy of cancer therapies, including immunotherapy (Nature Comm 2013; Cancer Discovery 2016; Science Translational Medicine 2017; PNAS 2019, 2020). A phase II trial (NCT01821729) led by my clinical collaborators provided compelling evidence in support of this emerging concept for improving the treatment outcome for patients with pancreatic ductal adenocarcinoma – a uniformly fatal disease (JAMA Oncology 2019).
Rakesh K. Jain receives National Medal of Science at the White House! ->Click here for the webcast. 
Lab News
Breast cancer can spread—or metastasize—to many different parts of the body, but it’s not well understood why tumors grow better in some organs than others. We explored whether the nutrients available in different tissues help determine where cancer spreads. Using mouse models and advanced metabolic profiling, we measured nutrient levels across several organs and tested how depriving cancer cells of specific nutrients affected their ability to form metastases.
Surprisingly, we found that no single nutrient explains why breast cancer grows in one organ and not another. Instead, a combination of multiple nutrients and cancer cell characteristics work together to determine where tumors can thrive.
See the MGB Research Spotlight HERE
See the article HERE.
How A Fringe Idea Led To Lifesaving Cancer Treatments
In cancer research, the “seed and soil” hypothesis posits that the tumor is like a seed of misbehaving cells taking root in the body. Whether it grows—and where it grows—depends on the conditions, or soil. Since this hypothesis was proposed more than 100 years ago, most research and treatments have focused on the seed, or tumor.
For nearly 50 years, Rakesh Jain has been studying the soil. But in a seed-focused field, his work was seen as wasteful and radical. Now, that very same research has led to seven FDA-approved treatments for diseases including lung and liver cancer, and earned him a National Medal of Science in 2016. Host Flora Lichtman talks with Jain about how his fringe idea led to lifesaving cancer treatments.
Guest: Dr. Rakesh K. Jain studies the biology of tumors at Harvard Medical School and Massachusetts General Hospital as a professor of radiation oncology.
Transcripts for each episode are available within 1-3 days at sciencefriday.com.
"CAR-T cells are T cells that have been extracted from a patient, modified in a laboratory to recognize and target cancer cells then infused back into the patient. They have revolutionized the treatment of many blood cancers, but have not yet benefited patients with solid tumors, such as lung, breast, colorectal or brain tumors. By developing a sophisticated mathematical model, we demonstrated that normalizing tumor blood vessels — which means transforming the abnormal, disorganized vasculature of a tumor into a more normal, structured and functional state — can improve the efficacy of CAR-T therapy and reduce the required therapeutic dose by about fivefold."
"Each researcher selected has authored multiple Highly Cited Papers that rank in the top 1% by citations for their field(s) and publication year in the Web of Science Core Collection over the past 11 years — indicating exceptional influence in their respective disciplines."
Brain tumor patients with glioblastoma (GBM) face extremely limited treatment options and show poor responses to current immunotherapies. New treatment strategies are desperately needed.
Wnt signaling is a crucial cell communication process that regulates various aspects of stem cell behavior. It is also pivotal in the generation of GBM and contributes to treatment resistance.
In this new study, we identify Wnt7b, which is highly expressed in GBM patients, as a previously unknown determinant of resistance to immune checkpoint blockers.
Jain Lab Team
Former Team Members
Current Research
Cancer metastasis is a major contributor to patient morbidity and mortality1, yet the factors that determine the organs where cancers can metastasize are incompletely understood. Here we quantify the absolute levels of 124 metabolites in multiple tissues in mice and investigate how this relates to the ability of breast cancer cells to grow in different organs. We engineered breast cancer cells with broad metastatic potential to be auxotrophic for specific nutrients and assessed their ability to colonize different tissue sites. We then asked how tumour growth in different tissues relates to nutrient availability and tumour biosynthetic activity. We find that single nutrients alone do not define the sites where breast cancer cells can grow as metastases. In addition, we identify purine synthesis as a requirement for tumour growth and metastasis across many tissues and find that this phenotype is independent of tissue nucleotide availability or tumour de novo nucleotide synthesis activity. These data suggest that a complex interplay between multiple nutrients within the microenvironment dictates potential sites of metastatic cancer growth, and highlights the interdependence between extrinsic environmental factors and intrinsic cellular properties in influencing where breast cancer cells can grow as metastases.
The spread of cancer cells from primary tumors to regional lymph nodes is often associated with reduced survival. One prevailing model to explain this association posits that fatal, distant metastases are seeded by lymph node metastases. This view provides a mechanistic basis for the TNM staging system and is the rationale for surgical resection of tumor-draining lymph nodes. Here we examine the evolutionary relationship between primary tumor, lymph node, and distant metastases in human colorectal cancer. Studying 213 archival biopsy samples from 17 patients, we used somatic variants in hypermutable DNA regions to reconstruct high-confidence phylogenetic trees. We found that in 65% of cases, lymphatic and distant metastases arose from independent subclones in the primary tumor, whereas in 35% of cases they shared common subclonal origin. Therefore, two different lineage relationships between lymphatic and distant metastases exist in colorectal cancer.
Angiotensin system inhibitors (ASIs) can improve prognosis in multiple cancer types, including pancreatic ductal adenocarcinoma (PDAC). However, no study has examined the effect of ASIs alone or combined with adjuvant chemotherapy in resected PDAC patients.<br /><br />Experimental Design: We performed an analysis of the records of ASI users and non-user patients with PDAC seen at Massachusetts General Hospital between January 2006 and December 2010. To identify mechanisms of ASIs in PDAC, we performed RNA-Seq of resected primary lesions. <br /><br />Results: 794 consecutive patients were included. In 299 resected patients, ASI-users experienced longer overall survival (OS) in both univariate (median OS: 36.3 vs. 19.3 months, p=0.011) and adjusted multivariate (HR, 0.505; 95%CI, 0.339 – 0.750; p=0.001) analyses. Propensity score adjusted analysis also showed a longer median OS for chronic ASI-users. In unresected patients, the beneficial effect of ASIs was significant in patients with locally advanced disease, but not in metastatic patients. RNA-Seq analysis revealed in tumors of ASI-users (lisinopril) a normalized extracellular matrix, a reduced expression of genes involved in PDAC progression (e.g. WNT and Notch signaling) and an increased expression of genes linked with the activity of T cells and antigen-presenting cells. Finally, chronic use of ASI was associated with a gene expression signature which is predictive of survival in independent validation cohorts.<br /><br />Conclusions: In patients with non-metastatic PDAC, chronic ASI use is associated with longer OS independently of chemotherapy. Our RNA-Seq analysis suggests that ASI reduce the malignant potential of cancer cells and stimulate the immune microenvironment in primary PDAC.
It remains unclear how obesity worsens treatment outcomes in patients with pancreatic ductal adenocarcinoma (PDAC). In normal pancreas, obesity promotes inflammation and fibrosis. We found in mouse models of PDAC that obesity also promotes desmoplasia associated with accelerated tumor growth and impaired delivery/efficacy of chemotherapeutics through reduced perfusion. Genetic and pharmacological inhibition of angiotensin-II type-1 receptor (AT1) reverses obesity-augmented desmoplasia and tumor growth and improves response to chemotherapy. Augmented activation of pancreatic stellate cells (PSCs) in obesity is induced by tumor-associated neutrophils (TANs) recruited by adipocyte-secreted IL-1ß. PSCs further secrete IL-1ß, and inactivation of PSCs reduces IL-1ß expression and TAN recruitment. Furthermore, depletion of TANs, IL-1ß inhibition, or inactivation of PSCs prevents obesity-accelerated tumor growth. In pancreatic cancer patients, we confirmed that obesity is associated with increased desmoplasia and reduced response to chemotherapy. We conclude that crosstalk between adipocytes, TANs, and PSCs exacerbates desmoplasia and promotes tumor progression in obesity.
Polarization of tumor-associated macrophages: a novel strategy for vascular normalization and antitumor immunity.
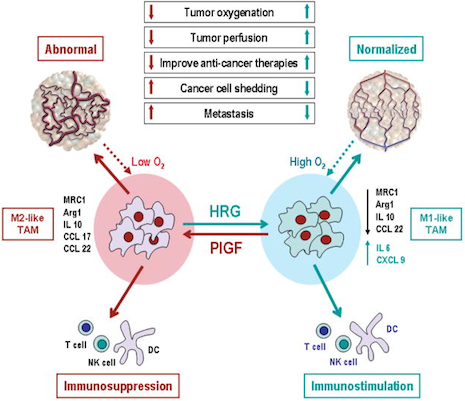
TAMs with M2-like phenotype lead to abnormal tumor vasculature by producing angiogenic factors, such as PlGF, and M2-cytokines, such as IL10 and CCL22. In addition, M2-cytokines suppress immune cell functions. Elevated levels of HRG polarize TAMs away from an M2-like phenotype to normalize tumor vessels and activate anti-tumor immunity. By fortifying tumor vessels, vascular normalization may decrease shedding of metastatic cells into circulation resulting in decreased metastasis. Normalized vessels may also facilitate delivery of drugs and immune cells. Reduction in hypoxia – known to increase resistance to radiation and a number of therapeutics – also sensitizes tumors to various therapies and decreases selection pressure for more malignant clone, and promotes M1-like TAM phenotype. All these effects of HRG treatment may result in decreased tumor growth and metastasis and increased efficacy of various therapies. PlGF deletion in macrophages can phenocopy many effects of HRG treatment. (Schematics of abnormal and normalized tumor vasculature reproduced from Jain, Nat Med 7:987, 2001).
Rapid blood perfusion is critical for postimplantation survival of thick, prevascularized bioartificial tissues. Yet the mechanism by which implanted vascular networks inosculate, or anastomose, with the host vasculature has been unknown, making it difficult to develop optimized strategies for facilitating perfusion. Here we show that implanted vascular networks anastomose with host vessels through a previously unidentified process of "wrapping and tapping" between the engrafted endothelial cells (ECs) and the host vasculature. At the host-implant interface, implanted ECs first wrap around nearby host vessels and then cause basement membrane and pericyte reorganization and localized displacement of the underlying host endothelium. In this way, the implanted ECs replace segments of host vessels to divert blood flow to the developing implanted vascular network. The process is facilitated by high levels of matrix metalloproteinase-14 and matrix metalloproteinase-9 expressed by the wrapping ECs. These findings open the door to new strategies for improving perfusion of tissue grafts and may have implications for other physiologic and pathologic processes involving postnatal vasculogenesis.
